Theory of air compression 2
An air compression is a means by which one type of energy is converted to another. During this conversion certain losses occur because of the rise in temperature of the air as it compressed. In general practice, the air is stored in a receiver and heat is lost both in the receiver and pipe lines running to equipment. Since the rise in temperature of the air is a direct loss of energy. We want to keep it down to a minimum. The ideal method is to compress air isothermally but this is impossible in practice owing to lack of time necessary to affect transfer.
Water jackets and inter-cooling can be used to keep the temperature down. These have the effect of reducing the compression index (n) to something less than 1.4.
When air is compressed to a pressure to exceeding about 4 bar it is usual to compress it in stages, with intercooling between each stage. This considerably reduces the total amount of work required on the air.
For two stages compressing, the air is compressed in the first (low pressure) stage adiabatically from p1 to p2 and then enters the intercooler where it is cooled down to the original temperature. Its volume is thereby reduced to V2 which is on the isothermal line. This volume of air now enters the high pressure cylinder, and is compressed to the final pressure and volume (p3 and V3). The law of compression is assumed to be the same for both compressors, namely:
p Vn = C
The pressure of intercooling to give the minimum of work done is when:
p2 = sqrt(p1 x p3)
Compression may be done in three or more stages to reduce the amount of work. Multistage compression approaches isothermal compression as the number of stages is increased.
Water jackets and inter-cooling can be used to keep the temperature down. These have the effect of reducing the compression index (n) to something less than 1.4.
When air is compressed to a pressure to exceeding about 4 bar it is usual to compress it in stages, with intercooling between each stage. This considerably reduces the total amount of work required on the air.
For two stages compressing, the air is compressed in the first (low pressure) stage adiabatically from p1 to p2 and then enters the intercooler where it is cooled down to the original temperature. Its volume is thereby reduced to V2 which is on the isothermal line. This volume of air now enters the high pressure cylinder, and is compressed to the final pressure and volume (p3 and V3). The law of compression is assumed to be the same for both compressors, namely:
p Vn = C
The pressure of intercooling to give the minimum of work done is when:
p2 = sqrt(p1 x p3)
Compression may be done in three or more stages to reduce the amount of work. Multistage compression approaches isothermal compression as the number of stages is increased.
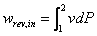

Comments