Theory of air compression
Air is not a perfect gas but for practical purpose the laws relative to perfect gases may be applied to it.
but for practical purpose the laws relative to perfect gases may be applied to it.
Boyle’s law states that: The absolute pressure of a gas varies inversely as the volume, provided the temperature remains constant.
states that: The absolute pressure of a gas varies inversely as the volume, provided the temperature remains constant.
p V = a constant
where: p = pressure in bar, V = volume in m3.
Charles’ law states that the volume of a gas under constant pressure, or the pressure of a gas under constant volume, varies as the absolute temperature. Therefore V varies as T, and p varies as T where T is the absolute temperature.
If the two laws are combined, we get:
p V / T = constant
The constant is usually denoted by R and therefore:
p V = R T
It can be shown that the value of the constant R applicable to air is 287.0 J/(kg K).
The relation between the pressure and volume of air during its expansion and compression may be represented by:
p Vn = R T
where ‘n’ has value which depends on the addition or subtraction of heat during the process.
When the temperature remains constant during compression or expansion they is said to be isothermal and the value of ‘n’ is one. In order to obtain pure isothermal compression it would be necessary to remove heat from the air at the same rate as heat is produced by the work done on the gas. When a gas expands and when no heat passes during expansion or contraction they is said to be adiabatic.
Boyle’s law
p V = a constant
where: p = pressure in bar, V = volume in m3.
Charles’ law states that the volume of a gas under constant pressure, or the pressure of a gas under constant volume, varies as the absolute temperature. Therefore V varies as T, and p varies as T where T is the absolute temperature.
If the two laws are combined, we get:
p V / T = constant
The constant is usually denoted by R and therefore:
p V = R T
It can be shown that the value of the constant R applicable to air is 287.0 J/(kg K).
The relation between the pressure and volume of air during its expansion and compression may be represented by:
p Vn = R T
where ‘n’ has value which depends on the addition or subtraction of heat during the process.
When the temperature remains constant during compression or expansion they is said to be isothermal and the value of ‘n’ is one. In order to obtain pure isothermal compression it would be necessary to remove heat from the air at the same rate as heat is produced by the work done on the gas. When a gas expands and when no heat passes during expansion or contraction they is said to be adiabatic.
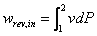
Comments